Pat Kellogg Roller, Children’s Science Specialist/Teacher/Retired
Welcome to Part 1 – KIDS CHEMISTRY!
For kids ages 8-12
INTRODUCTION//OVERVIEW
Why are we 17th in the developed nations in math and science education?
When elementary teachers reach the chapter about chemistry, they say, “Atoms, too abstract, too much vocabulary, too hard, don’t know chemistry, never had it, don’t know how to teach it, and besides it is just too dangerous! Quietly, softly, they move on. That attitude is highly contagious. Today, high school students avoid chemistry for the same reasons, but mostly because they think it is too hard. It doesn’t take a rocket scientist to understand why we are 17th in the developed nations in math and science education.
Many years of experience teaching kids ages 7-12, high interest “hands-on” activities and experiments taught me it is very effective. My students work cooperatively in Cooperative Learning Teams with 4-5 students in each team. Every student participates, every student is successful. If you’d like to know how to do that, I encourage you to check out CLASSROOM MANAGEMENT on this website. However; at the end of this Chemistry – Part 1 page, there is a list of what each team member is responsible for.
Problem solving skills which help students develop resilience come in handy as they face the frequent crisis inherent in CLIMATE CHANGE. Working in teams, supporting each other, is a good setting for their learning how to face difficulty, even make mistakes, and remain calm and able to cope…

There is a great wisdom in the old Chinese Proverb above. What children understand, they can use.
My students taught me these things:
1 . Kids are constantly changing; it is no great leap for them that earth stuff called MATTER is also.
2 . Kids are easily excited, so excited atoms are no surprise.
3 . They consider the atom a mystery, “a mystery they solve” with their tables, models, names, and games.
4 . “Surprises, wonder, amazing events, the EXPERIMENTS!”
Questions and comments from some of my students:
Will it blow up?
Will it put out the fire?
Will it pop to the ceiling, rattle the windows?
Can we make slime for Halloween?
Leave it in the fridge, will it grow mold?
Grow snowflakes for our Christmas tree? You must be kidding me!
Mystery powders? What are they? How do they act? When together, do they turn hot or turn cold?
Those mystery powders, they could be bad, they could be good, they could be just misunderstood!
They could cause warts, or a red, itchy rash! Ohhhhh – YUCK!
I dare you to touch them. If you will, I will.
What’s that red stuff in a bottle? It smells like dead cabbage!
My students and I, we did Kid’s Chemistry together in a spirit of adventure and fun. Every day was an exciting day! It made them feel smart, chemistry.
In these three parts of kid’s chemistry, I give you what we did, what we learned together, how I managed the classroom and the students. It’s all here free for the taking for those adults who are willing to step outside the box and get kids involved so they learn more of how our planet works!
Kid’s Chemistry offers easy, high interest, fun, inexpensive, chemistry activities and experiments for kids in grades 4-8. They actively use creative problem solving, develop chemistry vocabulary, and learn more about how our planet works by doing and understanding. They go home, repeat and continue experimenting because materials needed are in most kitchens or laundries.
The kids will observe, measure, classify, experiment, interpret, communicate information, and practice safety procedures in all science activities.
Part 1 – Into the Atom: Kid’s do ‘hands-on’ science activities to learn the parts of the atom, function of the each part and create models of atoms. The ‘Mystery Atoms’ game makes learning easy. They use the Periodic Table of the Elements to learn about earth stuff called matter. They can easily create models of any of the elements on the table. This will help them interpret the Periodic Table to see how the elements are arranged and to compare and contrast them. Part 1 is the most challenging since students have to read and understand the Periodic Table, as well as learn more vocabulary than the other two parts, but it is here that the groundwork is laid which is used in the other parts.
Part 2 – Moving Molecules, Physical Properties, Changes – This section is full of “exciting kids chemistry adventures” (experiments)which children experience. With involvement, kids begin to understand States of Matter, Properties of Matter, Moving Molecules, Physical and Chemical Changes and how ENERGY makes things move.
Part 3 – Mixtures, Compounds, Chemical Changes – Experiments and Activities – Amazing Mystery Powders, Baggie Bomb and Alkabomb, Balloon Blow-up and Dancing Weightlifters, Greatest Slime Experiment Ever, Grow a perfect Crystal Snowflake, The Incredible Edible Atom, and Calculate Molecular Weight.
Into the Atom – Part 1 – Teacher Directed
In chemistry, earth stuff is called matter. Chemistry is the study of matter and its interactions. All that exists on earth besides matter is energy and energy causes matter to change – to move.
Every particle of matter may be described by its physical properties; the way things look, feel, smell, or taste.
There are 92 natural kinds of earth stuff (matter). Each kind of matter is called an element. Hydrogen, nitrogen, gold, silver, and uranium are some of the elements.
Everything on earth is made from these 92 elements because they can change and combine in many different ways to make other earth stuff.
The smallest part of an element in Kid’s Chemistry is called an atom. We study atoms first. Atoms are made of three parts. They are protons (+), neutrons (o), and electrons (-).
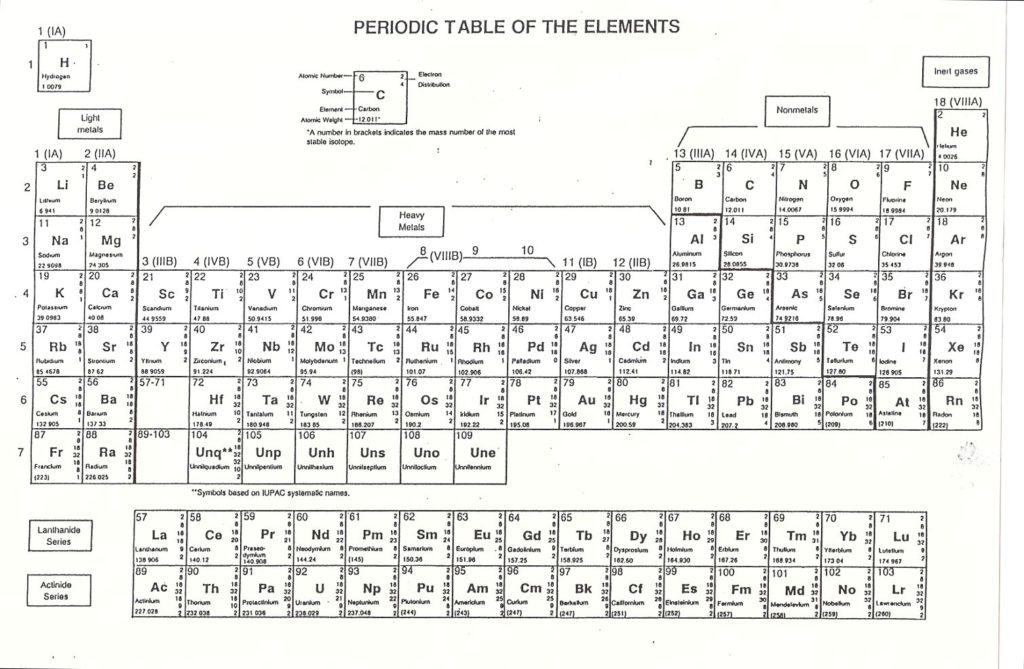
We can learn a lot about these 92 elements by studying the “Periodic Table of the Elements.”
(Note to Teacher/Parent) Distribute the Periodic Table of the Elements to the students. (Put master copy on the overhead projector.) To make the Periodic Table and other images grow larger just click on them.
Look at how the chart is labeled by groups: Light Metals, Heavy Metals, Non-Metals, Inert Gases.
Look at the Key at the top of the chart below the title: PERIODIC TABLE OF THE ELEMENTS. This is Carbon. The atomic number of Carbon is 6. Atomic Number is the number of protons in one atom of the element.
Look at the right upper corner. This is the ‘Electron Distribution.’ It shows 2 electrons in the first shell (K) and 4 electrons in the second shell (L). The electron shells or orbits are the pathways the electrons travel. Each shell or orbit is an energy level for a certain number of electrons. You might compare them to little moons orbiting a planet. There are 6 electrons. The number of protons and the number of electrons are the same number for an atom of an element.
In the middle of the ‘KEY’ box is the Symbol for the element, which is Carbon. In the lower left hand corner is the number 12.011. That is the Atomic Weight or mass of one atom. Round 12.011 to the nearest whole number (12).
To find the number of neutrons, subtract the Atomic Number(# of protons) from the Atomic Weight. We see 12-6=6. So there are six neutrons in this atom. The neutron is neutral and it contains most of the weight of the atom.
In an atom the protons (+) have a positive electrical charge. We use the (+) symbol for protons.
The neutrons (o) are neutral. They have no electrical charge. They make up almost all of the weight of the atom. Their symbol is (o).
The electrons (-) are high-energy particles with a negative electrical charge. In the elements, there are the same number of protons and electrons in every atom. The attraction between the protons and the electrons helps to hold the atom together.
The protons (+) and the neutrons (o) are in the NUCLEUS of the atom which is the center. The electrons (-) move in orbits around the nucleus.
Where else do you see this (+) and (-) symbols? (In electricity, in magnetism, and in the little batteries we put in our electronic games. It is always about ‘opposites attract.’)
Students may work well together in Cooperative Learning Teams at this point with teacher help as needed.
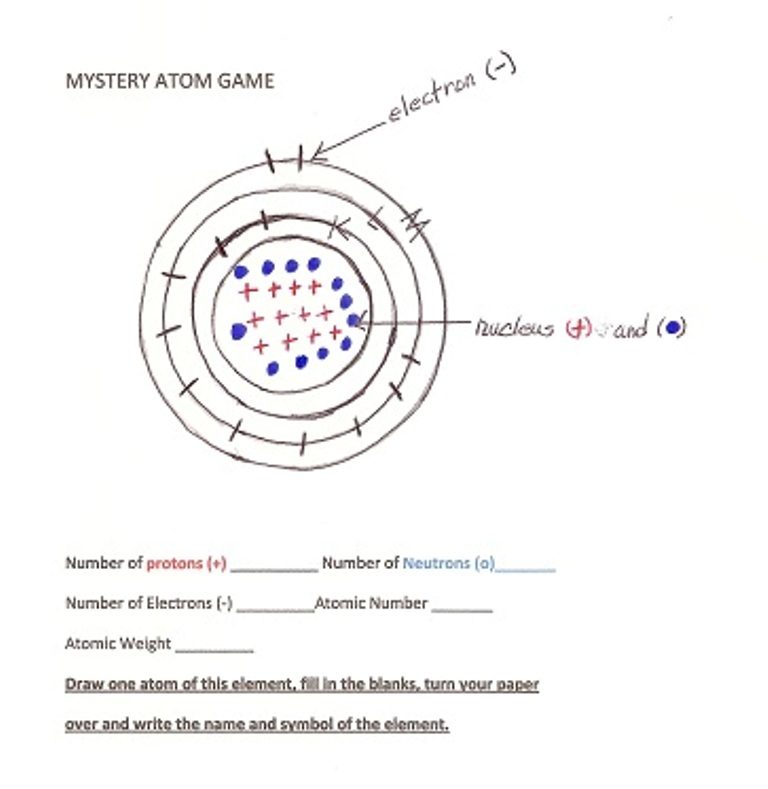
NOTE TO THE TEACHER/PARENT: Give each student the “MYSTERY ATOMS GAME.”
Fill out this chart together. This is an atom of Helium. See if the children can find Helium on the Periodic Table. It is in the upper right corner. Ask them what the Atomic Weight is (4.0026). Round it to the nearest whole number (4) to get the weight kids will use. Show them how to get the number of neutrons by subtracting the Atomic Number (Number of protons(+) from the Atomic Weight.
How many energy shells (orbits) does this element have? (1) What is Helium? How do we use it? (Inflate birthday balloons so they float up in the air, etc.)
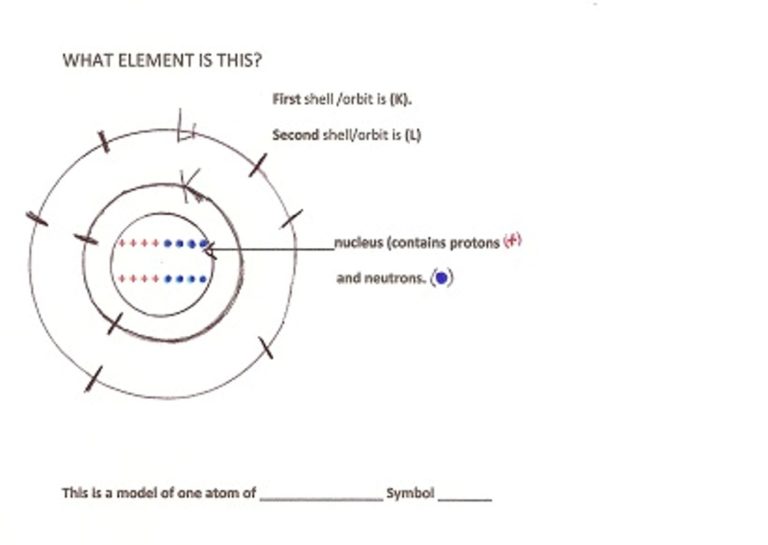
NOTE TO TEACHER/PARENT – Hand out the “WHAT ELEMENT IS THIS? Element is Oxygen.
Repeat the procedure with Oxygen you did with Helium with the kids. How is Oxygen different from Helium? (Has more particles.) How is it like Helium? (both are gases) How many energy shells does Oxygen have? (Shell K, and Shell L so there are two energy shells with electrons on each.)
Find Oxygen on the Periodic Table of the Elements, read its box and tell what each number means including rounding the weight to the nearest whole number.
Oxygen has many uses on our planet. We breathe it in so our bodies can change the food we eat to energy. Oxygen is plentiful in the air and in the earth’s crust.
NOTE TO TEACHER/PARENT – Hand out the next “WHAT ELEMENT IS THIS?”
Repeat the procedure for Oxygen. Have kids use Periodic Table to discover which element it is. Read the box for Magnesium. Compare and contrast the likenesses and differences between Helium, Oxygen, and Magnesium. Notice it has 3 energy shells and more particles.
Each additional electron shell after the first (K) is the next letter in the alphabet (K, L, M, N. N, O, P, Q, etc.) Notice the first shell (K) always has 2 electrons. Read from the 2 down to get the other shells.
Question – Since Magnesium has more particles, would it weigh more? To get the Atomic Weight, add the number of protons and neutrons together. Check this out on the Periodic Table to see if it matches to the nearest whole number.
At this point some of the kids will grasp the idea that the more particles in the atom, the greater the weight of the element. The Periodic Table is arranged from lightest to most heavy atoms.
Research: Where do we find magnesium and how is it used? (ask Google).
It’s time for a quiz to see if your students are ready to continue. Below is the one I use, after reviewing them while they take notes. Their notes are homework to study and prepare for test. Put the students into Cooperative Learning Teams and let them do the quiz together. Be sure they have the Periodic Table of the Elements to refer to.
QUIZ OVER PART 1 – CHEMISTRY -“ INTO THE ATOM “
Instruct students to number on notebook the questions and fill in the answers. You will only need 1 answer sheet from each group.
Part 1 – MATTER
1 . All matter is made of _____________, and matter is everywhere.
2 . All that exists on earth besides matter is __________ and ________ makes things change – move.
3 . How may every particle of matter be described? By its physical ________________.
4 . What are physical properties? The way things ________, __________, _________, _______.
5 How many elements occur naturally on earth? ______________.
6 . Elements are made of only ________ kind of atom.
7 . An_________ is the smallest part of an element that can exist.
Part 2 – INTO THE ATOM
8 . The ____________ charged particles in the atom are called protons.
9. The __________ charged particles in the atom are called electrons.
10. __________ have a positive (+) electrical charge.
11. Opposite charges always ________ each other.
12. __________ have a negative (-) electrical charge.
13. ___________ are neutral. They have no charge. They add _________ to the atom.
14. __________ Like charges always __________ each other.
15. ___________ makes things change – move.
16. ___________ is the study of matter and its interactions.
17. The center of the atom is called the __________.
18. What force holds the protons in the nucleus together? ____________ ___________.
19. Electrons weigh (little) or (much)? __________
20. What keeps the electrons in orbit around the nucleus of the atom? _________ ________ ________________
21. How many electrons can the first shell (K) hold? ____ The second shell (L) _________
22. Where in the atom are the protons and the neutrons? ____ _______ ________.
23. __________ are the heaviest part of the atom. They have no charge.
ANSWERS TO QUIZ
1. elements 2. energy, energy 3. physical properties 4. look, feel, smell, or taste 5. 92 6. one 7. atom 8.positive 9. negative 10. Protons 11. attract 12. Electrons 13. Neutrons 14. repel 15. Energy 16. chemistry 17. nucleus 18. nuclear force 19. little 20. attraction to protons 21. two, eight. 22. in the nucleus 23. neutrons
NOTE TO THE TEACHER: Emphasize neatness and legibility. Refer back to the “What Element Is This” lessons to demonstrate this. Kids should be in groups with half sheets of paper or cards. Have them look up some of the simple elements on the Periodic Table such as Sodium, Aluminum, Sulfur, Chlorine, and Argon. The children may decide which drawing each one will do. With 4 or 5 children in a group, this makes it quick and easy to get a drawing of each of the atoms. Each group produces 4 or 5 drawings.
Have them put the name and symbol of the element on the back of the drawing of the atom leaving the front drawing without the name or symbol. Let them use the Periodic Table as needed.
When each group has at least 4 good drawings, you can play the “MYSTERY ATOMS GAME” to see how many of the drawings they can identify. Play it like a card game. If you do this more than once, you will be amazed with what they can do. Always have the Periodic Table handy.
OVER THE TOP ACTIVITY – Kids can make mobiles of atoms in three dimensions using tiny cotton balls, taped or pasted on each electron energy shell for the electrons, keeping the nucleus with the protons and neutrons as a single drawing. Then put it together for a mobile of an atom. It makes an exciting and colorful room to enter.
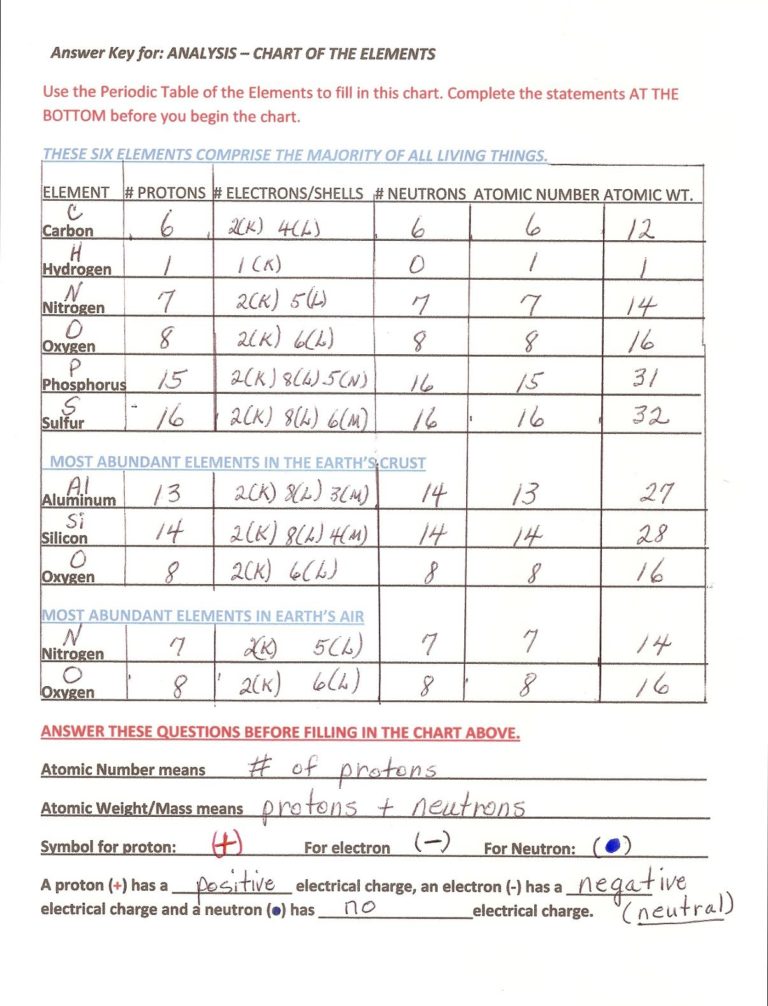
Point out to the kids that the chart is divided into three groups of elements: They are: These Six Elements Comprise the Majority of All Living Things, Most Abundant Elements in the Earth’s Crust, and Most Abundant Elements in Earth’s Air. Ask the kids to name the elements in each category.
This is actually a review of all you have taught so far about atoms. Encourage kids to help each other. Be sure to check charts for accuracy.
Challenge groups to draw a model of an atom of each of the elements listed in the analysis chart. Each child would draw one atom. Some they have already drawn.
NOTE TO TEACHER: Pass out one sheet per group of “MYSTERY ATOM GAME – Getting Bigger.” Be sure they have plenty of paper for drawings.
MYSTERY ATOM GAME – Getting Bigger
When the atom of an element has more particles in it does it weigh more or less? ______ How do you know?
To help you answer the question, draw one atom I describe; then compare it to your other atom drawings. How are they alike, different?
Use your Periodic Table of the Elements to find this element.
DESCRIPTION: 79 protons, 118 neutrons, 79 electrons in Six shells/orbits. They are: 2(K), 8(L), 18(M) 32(N)18(O) 2(P). Hint: You will need a full
sheet of paper.
What is the name and symbol for this element? _______________
What is the Atomic number: _____ Atomic weight ________?
Challenge: Draw one silver atom. Draw one uranium. Be very neat. (You need 4 pieces of paper taped together to do this one. Why? Is this element heavy or light weight?
Each student’s job in a Cooperative Group in the Lab
The GOAL is that the activity will be completed in such a manner that everyone will have an opportunity to participate, to share, and to learn.
Principal Investigator: This person will assume responsibility for team safety, following procedures in sequence, involving everyone in the group, completing the tasks and asking teacher questions as needed.
Recorder/Reporter – This person will assume responsibility for gathering experimental data from group members, doing necessary math and reporting results when requested.
Materials Manager – This person will assume responsibility for: picking up needed materials for the group, disbursing materials properly to group members and returning materials at the end of the activity.
Maintenance Director – This person will assume responsibility for cleaning up lab/activity area as needed and assisting materials manager as needed.
Observer: This person will assume responsibility for checking to see how well team members participated and giving an overview of how well the activity was conducted in terms of following directions, recording data, finishing the task and working cooperatively in clean-up.
End of – Part 1 Kid’s Chemistry.
PART 2 – KID’S CHEMISTRY AND PART 3 – KID’S CHEMISTRY continues this chemistry adventure sequentially.